Medical


Elastron medical TPE compounds meet special needs of various medical device applications. Medical TPE grades are tested according to USP ‘88’ for their in vivo biocompatibility and ISO 10993-5, USP ‘87’ for their in vitro cytotoxicity. Elastron has USP Class VI approved compounds.
Elastron medical TPE compounds also meet requirements of European Pharmacopoeia monographs 3.2.8 sterile single-use plastic syringes and 3.2.9 rubber closures for containers for aqueous parenteral preparations for powders and for freeze-dried powders. Elastron medical TPE compounds are sterilizable with gamma irradiation, ethylene oxide (EtO) and steam.
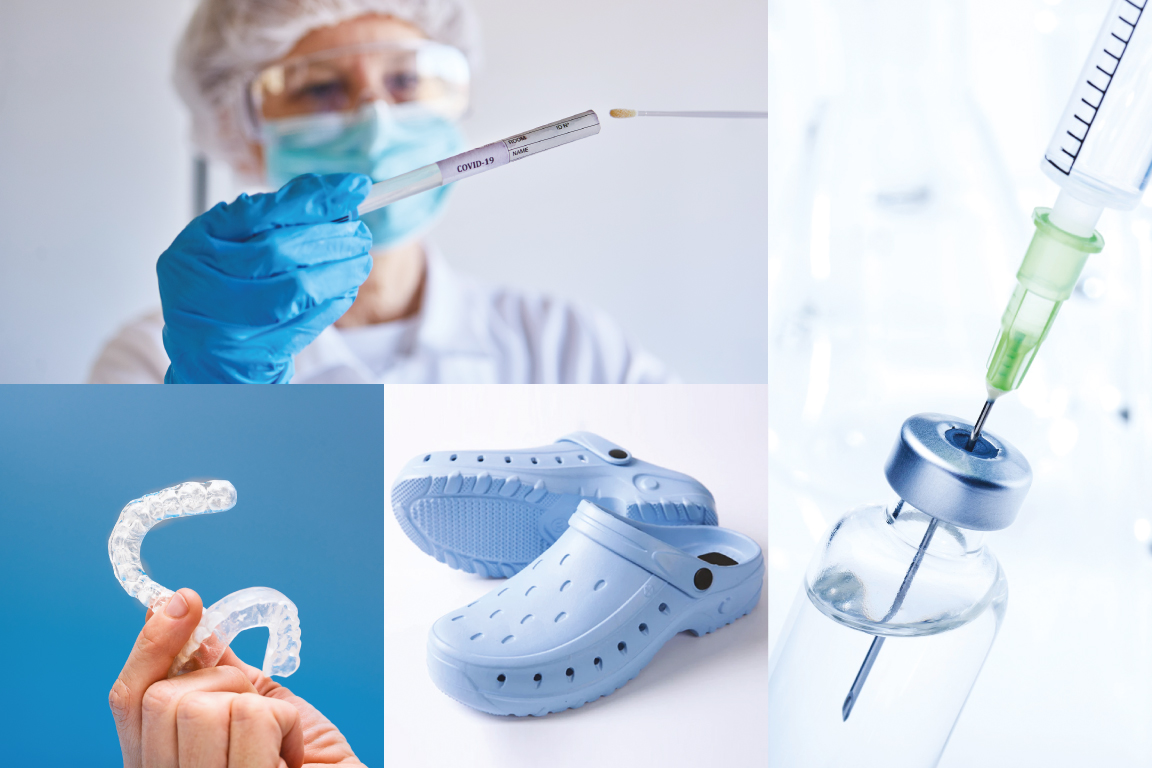
Segments & Important Applications
DISPOSABLE MEDICAL GOODS
- Brush of throat swabs
MEDICAL EQUIPMENT & DEVICES
- Dental guards for bruxism disease
- Medical tubes
- Urine catheter grips
MEDICAL FOOTWEAR
- Antibacterial shoes
- Antistatic hospital clogs
- Hospital clogs
MEDICAL TIPS & GASKETS
- Infusion bottle caps
- Syringe gaskets
- Medicine bottle caps
TPE PRODUCTS
TPV - Elastron V
SEBS - Elastron G
SBS - Elastron D
Key Features of Elastron TPE Grades for Medical Applications
- Permanent antistatic grades available up to 10⁸ ohm/square
- Selected compounds comply with recognized medical standards
- Availability of translucent compounds
- Free of latex, PVC, phthalates, and heavy metals
- Sterilizable by using common methods
- Good re-sealing properties
Please click for Elastron TPE Marketing Brochure.